Most of students search over Google for Haryana Board (HBSE) Important Questions 2026. Here is the Main reason because HBSE Board Says that in HBSE Exam 2026 (last 3 Years of Questions will Repeat) so that here are the selected List of Questions of Haryana Board For Class 9.
HBSE Class 9 Science Important Question Answer 2026
Chapter 1 – Matter in Our Surroundings
Q1. What do you know about sublimation?
Ans – Sublimation is the process in which a substance transitions directly from a solid to a gas phase, bypassing the liquid state. Or when solid directly convert into gas. Example – carbon dioxide
Q2. What is reason that we can get the smell of perfume sitting several metres away?
Ans – Because of diffusion, perfume molecules travel through the air and eventually reach your nose, allowing you to smell it even from several metres away. Perfume molecules evaporate and mix with air. These molecules diffuse from high concentration to low concentration areas.
Q3. Arrange the following substances in order of increasing force of attraction between their particles: (a) Water. (b) Sugar, (c) Oxygen, (d) Chlorine
Ans – We know that, The amount of force of attraction in different state of matter in increasing order is as follows gas–liquid–solid. So arrangement is as : Oxygen–chlorine–water–sugar.
Q4. Why do we see water droplets on the outer surface of a glass containing ice–cold water ?
Ans –Water droplets form on the outer surface of glass containing icecold water due to the condensation of water vapour in the air of water when it contacts the cold surface of the glass.
Q5. How does the water kept in an earthen pot (matka) become cool during summer ? Most Important
Ans – Water in an earthen pot (matka) becomes cool during summer due to the process of evaporation through its porous walls, which absorbs heat from the water inside and lowers its temperature.
Q6. The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its
Ans – The melting point is defined as the temperature at which a solid changes into a liquid at atmospheric pressure.
Q7. What will be the physical state of water at 25°C?
Ans – Water can exist in three physical states: solid, liquid, and gas. The state of water at different temperatures is determined by the kinetic energy of its molecules. At 25 °C, water is a liquid because the temperature is above its freezing point (0 °C) and below its boiling point (100 °C).
Q8. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Ans – A saucer has a larger surface area than a cup, which allows for faster evaporation of the liquid. This increased evaporation rate leads to quicker cooling of the tea or milk, making it more comfortable to sip.
Q9. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Ans – A diver is able to cut through water in a swimming pool. This shows that the particles of water have intermolecular space and has less force of attraction.
Q10. What are the characteristics of the particles of matter?
Answer. The characteristics of the particles of matter are:
(1) Particles have intermolecular space.
(2) Particles have intermolecular force.
(3) Particles of matter are moving continuously.
Q11. What type of clothes should we wear in summer?
Ans – We should wear light coloured cotton clothes in summer. Light colour because it reflects heat. Cotton clothes because it has pores in it, which absorbs sweat and allows the sweat to evaporate faster thereby giving cooling effect.
Q12. Give two reasons to justify (a) water at room temperature is a liquid. (b) an iron almirah is a solid at room temperature.
Ans – (a) Water at room temperature is a liquid because its freezing point is 0°C and boiling point is 100°C.
(b) An iron almirah is a solid at room temperature because melting point of iron is higher than room temperature.
Q13. Why is ice at 273 K more effective in cooling than water at the same temperature?
Ans – Ice at 273 K will absorb heat energy or latent heat from the medium to overcome the fusion to become water. Hence the cooling effect of ice is more than the water at same temperature because water does not absorb this extra heat from the medium.
Q14. Why does a desert cooler cool better on a hot dry day?
Ans – A desert cooler works by evaporative cooling, and it cools better on hot, dry days because low humidity and high temperatures significantly speed up water evaporation, which absorbs more heat from the air, creating a stronger cooling effect. The dry air readily accepts water vapor, allowing the water in the cooler to evaporate quickly, taking heat away and chilling the air that gets blown into the room.
Chapter 2 – Is Matter Around Us Pure
Q1. How are solution, suspension and colloid different from each other ?
Ans –
- Solutions are mixtures where the solute fully dissolves in the solvent, like sugar in water.
- Suspensions have larger particles that don’t dissolve and will settle over time, like oil in water.
- Colloids have particles that don’t settle, and light passes through them due to larger size, like milk.
Q2. Which are pure substances? Ice, Fe, Hg, Air, Sugar, River water
Ans – Ice, Fe, Hg, Sugar
Q3. Which of the following are chemical or physical changes?
(A) Cooking of food
(B) Melting of butter in a pan
(C) Boiling of water to form steam
(D) Burning of paper and wood
Ans – Cooking– A chemical change because raw ingredients change into something new, edible and a substance.
physical changes– Melting wax– Melting of butter in a pan– Boiling of water to form steam –
Q4. Name the technique to separate :
(i) Butter from curd
(ii) Camphor from salt
Ans – (i) Butter can be separated from curd by centrifugation.
(ii) Camphor can be separated from salt by sublimation.
Q5. What is a Solution ? Write properties of a solution.
Ans – A homogeneous mixture of two or more substances where the size of the particle is smaller than 1 nm is called the solution.
Properties
- Homogeneous Mixture: Solutions are homogeneous mixtures, meaning the components are uniformly distributed throughout the solution.
- Particles: The particles in a solution are smaller than 1 nm in diameter, making them undetectable by the naked eye.
- Stability: Solutions are stable, meaning they do not settle out or separate over time.
- Color: Solutions can be colored, but they are typically clear and transparent.
- Separation: Solutions cannot be separated by filtration due to the small size of the solute particles.
Q6. Write Four differences between Mixture and Compound. Most Important
Ans – Mixtures and compounds differ in composition, properties, methods of separation, and the nature of their components.
| Mixture | Compound |
|
|
|
|
|
|
|
|
Q7. List the points of differences between homogeneous and heterogeneous mixtures.
Ans –
| Homogeneous Mixtures | Heterogeneous Mixtures |
| It has uniform composition. | It does not have a uniform composition. |
| No visible boundaries of separation. | Shows visible boundaries of separation. |
| They consist of only one phase. | They consist of more than one phase. |
| Example – Sugar + Water → Sugar Solution | Example – Sugar + Sand |
Q8. What type of mixtures are separated by the technique of crystallisation?
Ans – Crystallisation technique is used to purify solid with some impurities in it. Example: Salt from sea–water.
Q9. Write the steps you would use for making tea. Use the words, solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Ans – 1. Take a cup of water in a container as solvent and heat it.
2. Add sugar in it which is solute. Heat it till all sugar dissolves.
3. You get a solution of water and sugar.
4. Sugar is soluble in water completely.
5. Add half a tea–spoon of tea–leaves, it is insoluble in water.
6. Boil the content, add milk which is also soluble in water, boil again.
7. Filter the tea with the help of strainer, the tea collected in cup is filtrate and the tea leaves collected on the strainer is residue.
Q10. How would, you confirm that a colourless liquid given to you is pure water?
Ans – By finding the boiling point of a given colourless liquid. If the liquid boils at 100°C at atmospheric pressure, then it is pure water. This is because pure substances have fixed melting and boiling point.
Q11. A suspension is a ……….Mixture.
Ans – heterogeneous
Q12. Differentiate between physical and chemical changes with examples.
Ans –
| Physical Change | Chemical Change |
|
|
|
|
|
|
|
|
Chapter 3 – Atoms and molecules
Q1. What is ion ? Give its example.
Ans – An ion is an electrically charged atom or group of atoms formed by the loss or gain of one or more electrons.
Q2. What are polyatomic ions ? Give examples. Most Important
Ans – Polyatomic ions are ions made of two or more atoms that are covalently bonded together and carry an overall charge. They behave like single ions in chemical reactions.
Examples of polyatomic ions:
- Nitrate:
- Sulphate:
- Carbonate:
- Ammonium:
- Hydroxide: OH⁻
- Phosphate:
- Bicarbonate (hydrogen carbonate):
Q3. Write the law of definite proportions.
OR
Write the Law of Constant Proportion. Most Important
Ans – The law of constant composition states that the chemical compounds consist of the elements which are present in a fixed ratio by their mass. This means that any given pure sample of the compound
Q4. Why is it not possible to see an atom with naked eyes?
Ans – Atoms are too small to be seen with the naked eye, as their size is on the order of nanometers, far beyond the resolution of human vision.
Q5. Define and explain molecular formula and write molecular formula of the following compounds:
(i) Sodium Oxide
(ii) Sodium Sulphide
(iii) Aluminium Sulphate
(iv) Magnesium Oxide
(v) Aluminium Oxide
(vi) Calcium Carbonate
(vii) Aluminium chloride
(viii) Magnesium hydroxide
(ix) Sodium carbonate
Ans – A molecular formula shows the actual number and types of atoms present in a molecule of a compound. It tells you which elements are present and how many atoms of each element combine to form one molecule or formula unit.
Q6. Below are the molecular formulas of the given compounds:
(i) Sodium oxide –Formula: Na₂O
(ii) Sodium sulphide –Formula: Na₂S
(iii) Aluminium sulphate –Formula: Al₂(SO₄)₃
(iv) Magnesium oxide –Formula: MgO
(v) Aluminium oxide –Formula: Al₂O₃
(vi) Calcium carbonate –Formula: CaCO₃
(vii) Aluminium chloride –Formula: AlCl₃
(viii) Magnesium hydroxide –Formula: Mg(OH)₂
(ix) Sodium carbonate–Formula: Na₂CO₃
Q7. Calculate molecular mass of the following compounds: ZnCO3, ZnO, CaSO4, Na2S, H2SO4, and HNO3
(Atomic Masses : Zn = 65.04, C = 12.04, O = 16.04, Ca = 40.04, S = 32.04, Na = 23.04, H = 1.04, N = 14.04)
Ans – Here are the molecular–mass calculations.
1. ZnCO₃
Zn = 65
C = 12
O₃ = 16 × 3 = 48
Molecular mass = 65 + 12 + 48 = 125 u
2. ZnO
Zn = 65
O = 16
Molecular mass = 65 + 16 = 81 u
3. CaSO₄
Ca = 40
S = 32
O₄ = 16 × 4 = 64
Molecular mass = 40 + 32 + 64 = 136 u
4. Na₂S
Na₂ = 23 × 2 = 46
S = 32
Molecular mass = 46 + 32 = 78 u
5. H₂SO₄
H₂ = 1 × 2 = 2
S = 32
O₄ = 16 × 4 = 64
Molecular mass = 2 + 32 + 64 = 98 u
6. HNO₃
H = 1
N = 14
O₃ = 16 × 3 = 48
Molecular mass = 1 + 14 + 48 = 63 u
Q8. Write the chemical formulae of the following:
(i) Magnesium Chloride
(ii) Copper Nitrate
(iii) Hydrogen Sulphide
(iv) Calcium chloride
(v) Aluminium Chloride
(vi) Sodium Oxide
(vii) Aluminium Chloride
Ans – (i) Magnesium chloride
Magnesium ion: Mg²⁺
Chloride ion: Cl⁻
Formula: MgCl₂
(ii) Copper nitrate
Copper commonly forms Cu²⁺
Nitrate ion:
Formula: Cu(NO₃)₂
(iii) Hydrogen sulphide
Hydrogen ion: H⁺
Sulphide ion: S²⁻
Formula: H₂S
(iv) Calcium chloride
Calcium ion: Ca²⁺
Chloride ion: Cl⁻
Formula: CaCl₂
(v) Aluminium chloride
Aluminium ion: Al³⁺
Chloride ion: Cl⁻
Formula: AlCl₃
(vi) Sodium oxide
Sodium ion: Na⁺
Oxide ion: O²⁻
Formula: Na₂O
(vii) Aluminium chloride
Aluminium ion: Al³⁺
Chloride ion: Cl⁻
Formula: AlCl₃
Q9. Give the names of the elements present in the following compounds:
(i) Quick lime
(ii) Hydrogen bromide
(iii) Slaked lime
(iv) Baking Powder
Ans – (i) Quick lime (chemical formula: CaO)
Elements present: Calcium and Oxygen
(ii) Hydrogen bromide (chemical formula: HBr)
Elements present: Hydrogen and Bromine
(iii) Slaked Lime (Chemical Formula: Ca(OH)₂)
Elements Present : Calcium, Oxygen and Hydrogen.
(iv) Baking Powder (Chemical Formula : NaHCO3)
Elements Present : Sodium, Hydrogen, Carbon, Oxygen
Q10. Write down the names of compounds represented by the following :
(a) K2SO4
(b) CaCl2
(c) KNO3
(d) Al2(SO4)3
(e) K2SO4
(f) CaCO3
Ans– Here are the names of the given compounds:
(a) K₂SO₄ → Potassium sulphate
(b) CaCl₂ → Calcium chloride
(c) KNO3 → Potassium Nitrate
(d) Al₂(SO₄)₃ → Aluminium sulphate
(e) K₂SO₄ → Potassium Sulphate
(f) CaCO₃ → Calcium carbonate
Q11. Calculate the molecular mass of Nitric Acid (HNO3) .
Ans – To calculate the molecular mass of Nitric Acid (HNO₃):
Atomic masses:
H = 1
N = 14
O = 16
HNO₃ = (1 × 1) + (14 × 1) + (16 × 3)
= 1 + 14 + 48
= 63 u
Molecular mass of Nitric Acid = 63 u
Q12. Calculate the molecular masses of :
(a) C2H2
(b) Cl2
Ans – Let’s calculate the molecular masses step by step.
Atomic masses:
C = 12, H = 1, Cl = 35.5
(a) C₂H₂
C₂ = 12 × 2 = 24
H₂ = 1 × 2 = 2
Molecular mass = 24 + 2 = 26 u
(b) Cl₂
Cl₂ = 35.5 × 2 = 71 u
Molecular masses:
C₂H₂ = 26 u
Cl₂ = 71 u
Q13. Write chemical formula of Carbon tetrachloride.
Ans – CCl4
Q14. Explain the following:
(i) Atomic mass unit
(ii) Law of conservation of mass
Ans –
(i) Atomic mass unit (amu): Atomic mass unit is defined as one-twelfth (1/12th) of the mass of one atom of carbon-12. It is used to express the masses of atoms and molecules.
(ii) Law of conservation of mass: The law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. The total mass of reactants is equal to the total mass of products.
Chapter 4 – Structure of the Atom
Q1. Explain with examples: Most Important
(a) Atomic number
(b) Isotopes
(c) Mass Number
(d) Isobars
Ans –
(a) Atomic Number: The number of protons present in the nucleus of an atom is called the atomic number. Example: Hydrogen has atomic number 1 because it has 1 proton in its nucleus.
(b) Isotopes: Atoms of the same element having the same atomic number but different mass numbers are called isotopes. Example: Isotopes of hydrogen – Protium, Deuterium, and Tritium.
(c) Mass Number: The total number of protons and neutrons present in the nucleus of an atom is called the mass number. Example: Carbon-12 has a mass number of 12 (6 protons + 6 neutrons).
(d) Isobars: Atoms having the same mass number but different atomic numbers are called isobars.
Example: Calcium-40 and Argon-40.
Q2. Define mass number. Write the mass number of carbon.
Ans – Mass number is the sum of number of protons and number of neutrons present in any atom
On the periodic table the mass of carbon is reported as 12.01 amu.
Q3. If K and L shells of an atom are full then what would be the total number of electrons in the atom?
Ans – If the K and L shells of an atom are full, the total number of electrons in the atom would be 10. This is because the K shell can hold 2 electrons and the L shell can hold 8 electrons, resulting in a total of 2 + 8 = 10 electrons.
Q4. Write the distribution of electrons in carbon and sodium atoms.
Ans – The electron distribution in carbon is 2, 4, and in sodium is 2, 8, 1, reflecting their respective electron configurations of 1s² 2s² 2p² for carbon and 1s² 2s² 2p⁶ 3s¹ for sodium.
Q5. Name the three sub–atomic particles of an atom.
Ans – Proton, Neutron, Electron.
Q6. Write the distribution of electrons in Nitrogen and Magnesium.
Ans – nitrogen has 7 electrons. The first 2 go into the K shell, and the remaining 5 go into the L shell.
Sodium has 11 electrons. The first 2 fill the K shell, the next 8 fill the L shell, and the last 1 goes into the M shell.
Q7. If number of electrons in an atom is 8 and number of protons is also 8, then:
(i) What is the atomic number of the atom?
(ii) What is the charge on the atom?
Ans – (i) The a tomic number of the atom is 8, which is determined by the number of protons.
(ii) The charge on the atom is 0 (neutral), as the number of electrons equals the number of protons, resulting in no overall charge.
Q8. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Ans – According to Thomson’s model of an atom
(i) An atom consists of a positively charged sphere and the electrons are embedded in it,
(ii) The negative and positive charges are equal in magnitude. So the atom is electrically neutral.
Q9. What are the limitations of J.J. Thomson’s model of the atom?
Ans – According to J.J. Thomson’s model of an atom, the electrons are embedded all over in the positively charged spheres. But experiments done by other scientists showed that protons are present only in the centre of the atom and electrons are distributed around it.
Q10. On the basis of Rutherford’s model of an atom, which sub–atomic particle is present in the nucleus of an atom?
Ans – As per Rutherford’s model of an atom, the protons which are positively charged are present in the nucleus of an atom.
Q11. What are the limitations of Rutherford’s model of the atom?
Ans – According to Rutherford’s model of an atom the electrons are revolving in a circular orbit around the nucleus. Any such particle that revolves would undergo acceleration and radiate energy. The revolving electron would lose its energy and finally fall into the nucleus, the atom would be highly unstable. But we know that atoms are quite stable.
Q12. Describe Bohr’s model of the atom.
Ans – Bohr’s model of the atom
(1) Atom has nucleus in the centre.
(2) Electrons revolve around the nucleus.
(3) Certain special orbits known as discrete orbits of electrons are allowed inside the atom.
(4) While revolving in discrete orbits the electrons do not radiate energy.
(5) These orbits or shells are called energy levels.
(6) These orbits or shells are represented by the letters K, L, M, N or the numbers n = 1, 2, 3, 4
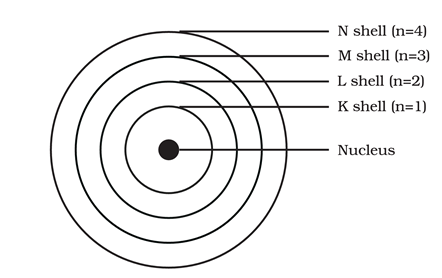
Q13. If z = 4 what would be the valency of the element? Also name the element.
Ans – If Z = 4, the element has atomic number 4.
The element is Beryllium (Be).
Electronic configuration of Be = 2, 2
It has 2 electrons in its outermost shell, so it has valency is 2.
Q14. Na+ has completely filled K and L shells. Explain.
Ans – Sodium (Na) has atomic number 11. Its electronic configuration is 2, 8, 1. When sodium loses one electron to form Na⁺, it becomes 2, 8. Thus, both the K shell (2 electrons) and L shell (8 electrons) are completely filled.
Q15. Explain Rutherford’s atomic model.
Ans – Ernest Rutherford was interested in knowing how the electrons are arranged within an atom. He conducted an experiment in which a thin gold foil was bombarded with fast moving alpha (α) particles.
- He selected a gold foil because he wanted a layer as thin as possible. The gold foil was about 1000 atoms thick.
- The particles used for bombarding the gold foil were doubly-charged helium ions. Since, they have a mass of 4 u, the fast-moving α particles have a considerable amount of energy.
- It was expected that α particles would be deflected by the sub-atomic particles in the gold atoms. Since, the α particles were much heavier than the protons, he did not expect to see large deflections.
But the α particle scattering experiment produced totally unexpected results.
The following observations were made:
(i) Most of the fast moving α-particles passed straight through the gold foil.
(ii) Some of the α-particles were deflected by the foil by small angles.
(iii) Surprisingly, one out of every 12000 particles appeared to rebound.
Conclusions:
(i) The first observation led to the conclusion that most of the atom is hollow.
(ii) The small angle deviation of the α particles confirms the presence of a positively charged centre called nucleus.
(iii) The third observation confirms that the nucleus of an atom is solid as the ray returns on its path and is very small in size because 1 out of 12000 rays returns.
Chapter 5 – The Fundamental Unit of Life
Q1. Make a comparison and write down ways in which plant cells are different from animal cells. Most Important
Ans –
| Plant Cell | Animal Cell |
| Cell wall is present. | Cell wall is absent. |
| Shape is usually fixed and rectangular. | Shape is usually irregular or round. |
| Chloroplasts are present for photosynthesis. | Chloroplasts are absent. |
| Large central vacuole is present. | Vacuoles are small or absent. |
| Food is stored in the form of starch. | Food is stored in the form of glycogen. |
| Mode of nutrition is autotrophic. | Mode of nutrition is heterotrophic. |
| Centrioles are absent. | Centrioles are present. |
| Cilia and flagella are usually absent. | Cilia and flagella may be present. |
Q2. Write the two differences between Prokaryotic cell and Eukaryotic cell.
Ans – Here are two key differences between prokaryotic and eukaryotic cells:
1. Nucleus:
- Prokaryotic cells do not have a true nucleus; their genetic material is free in the cytoplasm.
- Eukaryotic cells have a well–defined nucleus enclosed by a nuclear membrane.
2. Organelles:
- Prokaryotic cells lack membrane–bound organelles (like mitochondria, endoplasmic reticulum).
- Eukaryotic cells contain membrane–bound organelles that perform specific functions.
Q3. Write down the functions of nucleus.
Ans – Its primary role is to regulate gene expression, mediate replication, and coordinate cell activities like growth and metabolism.
Q4. Which organelle is known as the powerhouse of the cell ? And why ? Most Important
Ans – The mitochondrion is known as the powerhouse of the cell. Mitochondria produce energy in the form of ATP (adenosine triphosphate) through the process of cellular respiration, which the cell uses to perform all its vital functions. Without mitochondria, cells would not have the energy needed for growth, movement, or other metabolic activities.
Q5. Why are lysosomes known as suicide bags ? Most Important
Ans – Lysosomes are known as suicide bags because they contain digestive enzymes that can break down the cell’s own components. If a lysosome bursts accidentally inside the cell, its enzymes digest the cell’s organelles and cytoplasm, leading to the death of the cell.
This self–digestion is why they are called “suicide bags.” In short, lysosomes have the potential to destroy the very cell that contains them if their enzymes are released uncontrolled.
Q6. What is the meaning of cell in Latin ?
Ans – The word “cell” comes from the Latin term “cella” or “cellula,” which means “a small room”. It originally referred to a small room in a monastery or a hermit’s dwelling.
Q7. Where do the lipids and proteins constituting the cell membrane synthesized ?
Ans – Lipids and proteins constituting the cell membrane are synthesized in the endoplasmic reticulum (ER), with lipids produced in the smooth ER and proteins in the rough ER.
Q8. Draw a well labelled diagram of a plant cell.
Ans –
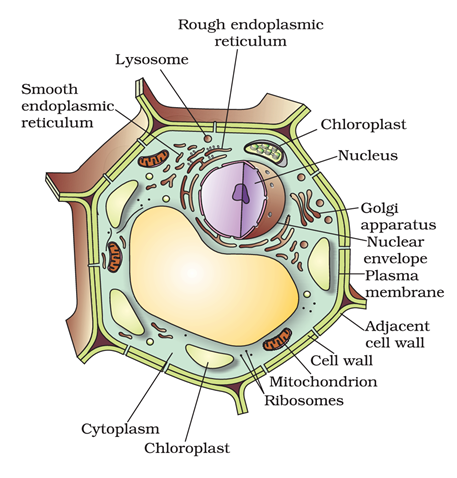
Synthesizes lipids, including phospholipids and cholesterol, which are essential for building cellular membranes.
Produces membrane–bound and secretory proteins, many of which are incorporated into the cell membrane or exported from the cell.
Q9. Who discovered cells, and how? Most Important
Ans – Robert Hooke discovered cells in 1665 while examining a thin slice of cork through a self–designed microscope. He saw that the cork resembled the structure of a honey comb consisting of many little compartments. These small boxes are called cells.
Q10. Why is the plasma membrane called a selectively permeable membrane? Most Important
Ans – It is called selectively permeable membrane because it allows the entry and exit of some substances, not all.
Q11. Where do the lipids and proteins constituting the cell membrane get synthesised? Most Important
Ans – Lipids and proteins are synthesised in ER [Endoplasmic Reticulum].
Q12. What is osmosis?
Ans – Osmosis is the process of movement of water molecule from a region of higher water concentration through a semi–permeable membrane to a region of lower water concentration.
Q13. Draw a well labelled diagram of a Animal cell.
Ans –
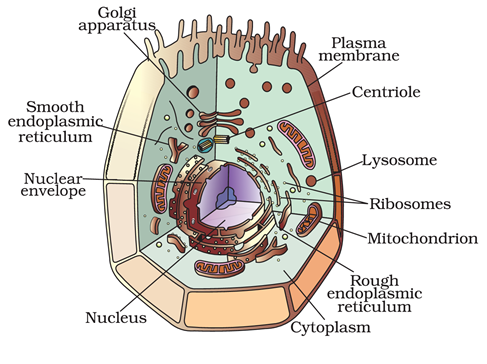
Q14. Nervous tissue is made of that receive and conduct impulses.
Ans – Neurons and neuroglia (also known as glial cells or glia).
Q15. Why is the cell called the structural and functional unit of life ?
Ans – The cell is called the structural and functional unit of life because:
- Structural unit: All living organisms are made up of cells, which form the basic building blocks of life.
- Functional unit: All vital life processes (like respiration, growth, reproduction, and excretion) take place within cells.
Hence, the cell is the smallest unit that can carry out all functions of life.
Q16. Name one organelle that contain its own genetic material.
Ans – Mitochondria
Q17. How does an Amoeba obtain its food?
Ans – Amoeba takes in food using temporary finger-like extensions of the cell surface which fuse over the food particle forming a food-vacuole . Inside the food vacuole, complex substances are broken down into simpler ones which then diffuse into the cytoplasm. The remaining undigested material is moved to the surface of the cell and thrown out.
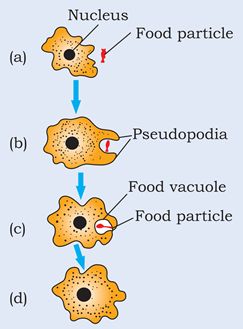
Chapter 6 – Tissues
Q1. Describe the role of epidermis in plants. Most Important
Ans – The epidermis in plants serves as the outermost protective layer, crucial for shielding internal tissues, regulating water loss, and enabling gas exchange.
Q2. Write three features of cardiac muscles. Most Important
Ans –
- The cardiac muscles are involuntary in function.
- Their functioning cannot be controlled by humans.
- They are involved in continuous rhythmic contraction and relaxation of the heart.
Q3. What are the Junctions of areolar tissue?
Ans – Areolar tissue are connective tissues found in animal. It is found between skin and muscles, around blood vessels and nerves and in the bone marrow. It fills the space inside the organs, supports internal organs and helps in the repair of tissues.
Q4. Describe the structure and functions of Areolar and Cartilage connective tissue.
Ans – description of areolar and cartilage connective tissues:
1. Areolar connective tissue
Structure:
- Loose connective tissue with loosely arranged fibers (collagen, elastic, and reticular) in a semi–fluid ground substance.
- Contains various cells: fibroblasts, macrophages, mast cells, and some white blood cells.
- Found beneath the skin, around blood vessels, nerves, and organs.
Functions:
- Provides support and cushioning to organs.
- Acts as a binding tissue connecting different tissues.
- Stores water and salts for surrounding tissues.
- Plays a role in inflammation and immunity due to presence of immune cells.
2. Cartilage connective tissue
Structure:
- Cells called chondrocytes are embedded in firm, flexible matrix containing collagen and elastic fibers.
- Covered by perichondrium (a connective tissue layer) which helps in growth and repair.
- Lacks blood vessels; nutrients diffuse through the matrix.
Functions:
- Provides support and shape to various body parts like nose, ear, and trachea.
- Reduces friction and absorbs shock in joints (hyaline cartilage in joints).
- Provides flexibility while maintaining strength (elastic cartilage).
Q5. Diagrammatically show the difference between the three types of Muscle Fibres. Most Important
OR
Draw a diagram of Smooth muscle.
Ans –
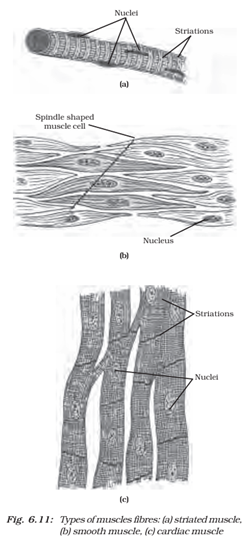
Striated Muscle Fibers: These are striated and under voluntary control, attached to bones, and responsible for body movements. They are long and cylindrical in shape.
Smooth Muscle Fibers: These are non–striated and involuntary, found in the walls of internal organs such as the stomach and blood vessels. They are spindle–shaped and control involuntary movements.
Cardiac Muscle Fibers: These are striated and involuntary, found only in the heart. They are branched and interconnected, allowing for synchronized contractions of the heart.
Q6. Name the following :
(a) Tissue that stores fat in our body.
(b) Connective tissue with a fluid matrix.
(c) Tissue present in the brain
Ans – (A) Adipose tissue is the tissue in humans that stores fat. It is made up of adipocytes, which are specialised cells. Its primary role is to store energy as fat while also acting as a shock absorber (cushion) and insulator.
(B) The connective tissue with a fluid matrix is blood.
- Blood is a type of connective tissue because it connects the body systems by transporting nutrients, gases, and wastes.
- Its matrix is called plasma, which is a fluid containing water, salts, proteins, and other substances.
- Blood also contains cells like red blood cells, white blood cells, and platelets suspended in the plasma.
(C) The tissue present in the brain is nervous tissue.
- Nervous tissue is made up of neurons (nerve cells) and neuroglia (supporting cells).
- Neurons transmit electrical signals throughout the body, allowing the brain to control and coordinate body functions.
- Neuroglia provide support, protection, and nourishment to neurons.
- The brain is largely composed of this tissue to perform its complex functions like thinking, memory, and coordination.
Q7. Define the neuron with diagram. Most Important
Ans –
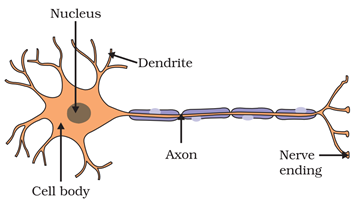
A neuron is a nerve cell that processes and transmits information through electrical and chemical signals in the nervous system
Q8. Write the three differences between parenchyma, collenchyma and sclcrenchyma.
OR
Differentiate between parenchyma, collenchyma and sclerenchyma on the basis of their cell wall. Most Important
Ans – Here are three key differences between parenchyma, collenchyma, and sclerenchyma:
| Feature | Parenchyma | Collenchyma | Sclerenchyma |
| Cell wall | Thin primary cell wall | Unevenly thickened primary cell wall | Thick, lignified secondary cell wall |
| Function | Storage, photosynthesis, and tissue repair | Provides flexible support to growing parts of the plant | Provides rigid support and mechanical strength |
| Cell condition | Living at maturity | Living at maturity | Usually dead at maturity |
These differences help plants maintain structure, flexibility, and strength depending on the tissue type.
Q9. Write the three functions of Areolar connective tissue ? Most Important
Ans – Three functions of areolar connective tissue are:
- Support and binding: It connects and holds different tissues and organs together.
- Cushioning and protection: It cushions organs and protects them from mechanical injury.
- Storage and transport: It stores water and salts and helps in the transport of nutrients and waste between tissues.
Q10. What are the functions of the stomata? Most Important
Ans – They play an important role in gas exchange, allowing plants to take in carbon dioxide for photosynthesis and release oxygen and water vapor.
Q11. Which tissue makes up the husk of coconut ?
Ans – The husk of a coconut is made up of sclerenchyma tissue.
- Sclerenchyma cells have thick, lignified cell walls, which provide hardness and mechanical strength.
- This makes the coconut husk tough, fibrous, and protective, helping the seed inside survive harsh conditions.
Q12. What are the constituent of Xylem ?
OR
How many types of elements together makeup the xylem tissue? Name them.
Ans – The xylem is a complex vascular tissue in plants that conducts water and minerals from roots to other parts. Its main constituents are:
- Tracheids: Long, tubular cells with thick lignified walls; help in water conduction and support.
- Vessels (or xylem vessels): Tube–like structures formed by end–to–end fusion of cells; allow efficient water transport.
- Xylem fibers: Thick–walled, elongated cells that provide mechanical support.
- Xylem parenchyma: Living cells that store food and help in lateral transport of water and minerals.
Together, these components provide conduction, support, and storage functions for the plant.
Q13. Name the tissue that transports food in plants. Most Important
Ans – Phloem
Q14. Name the tissue that transports Water in plants.
Ans – Xylem
Q15. Tissue that forms the inner lining of our mouth.
Ans – Epithelial tissue.
Q16. Name types of simple tissues.
Ans – The types of simple tissues are parenchyma, collenchyma, sclerenchyma and aerenchyma.
Q17. Name the regions in which parenchyma tissue is present.
Ans – In the pith of the roots and stems. When it contains chlorophyll, it is called chlorenchyma, found in green leaves. In aquatic plants, parenchyma contains large air cavities and help them to float. Such type of parenchyma is called aerenchyma.
Chapter 7– Motion
Q1. Define Acceleration. Write unit and formula of acceleration. Most Important
Ans – Acceleration refers to the rate of change in an object’s velocity over a period of time. This vector quantity is calculated using the average acceleration formula: Δv / Δt . The SI unit for acceleration is meters per second squared (m/s²) or kilometres per hour squared (km/h²).
Q2. Define average speed. Write the formula of average speed. Most Important
Ans – Average speed is defined as the total distance traveled divided by the total time taken to cover that distance.
Average Speed = Total distance covered ÷ Total time taken
Q3. A train starting from a railway station and moving with uniform acceleration attains a speed 40 km/hour in 10 minutes. Find its acceleration. Most Important
Ans – To find the acceleration of the train, use the formula:
Where: ![]()
initial speed (u) = 0
Final Speed (v) = 40km/h = ![]() = 11.11m/s
= 11.11m/s
Time (t) = 10 min = 10 x 60 = 600 second.
Apply it on formula,
![]() =
= ![]()
a = 0.0185 m/s2
Thus, the acceleration of the train is approximately 0.0185 m/s².
Q4. A racing car has a uniform acceleration of 4 metre/Sec2. What distance will it cover in 10 sec. after start ? Most Important
Ans – we know that, ![]()
Where,
Apply it on formula,
– 1.11 m/s2
![]()
s = 200m
Distance Covered = 200 meters
Q5. The brakes applied to a car produce an acceleration of 6 ms- 2 in the opposite direction to the motion. If the car takes 2 s to stop after the application of brakes, calculate the distance it travels during this time.
Ans – Acceleration a = 6 ms-2
Time t = 2s
Final velocity, v = 0
Step 1: Find Initial velocity using
V = u + at
0 = u + (-6)(2)
u = 12 m/s
Step 2 : Calculate distance travelled using
![]()
![]()
s = 24 – 12 = 12 m
Q6. A bus decreases its speed from 80 kmh–1 to 60 kmh–1 in 5S. Find the acceleration of the bus.
Ans – To find the acceleration of the bus, we use the formula for acceleration:
![]()
Where:
Initial Speed (u) = 80km.h = ![]() = 22.22m/s
= 22.22m/s
Final Speed (v) = 60 km/h = ![]() = 16.67 m/s
= 16.67 m/s
Apply the formula = ![]() =
= ![]()
a = ![]()
a = – 1.11m/s2
The acceleration of the bus is –1.11 m/s², indicating a deceleration.
Q7. Distinguish between speed and velocity.
Ans: The key distinctions are based on definition, direction, formula, and value range.
- Speed is defined as the distance travelled by an object in a given interval of time, and it does not have any direction. Velocity is the displacement of an object in a given interval of time and has a unique direction.
- Speed is a scalar quantity, while velocity is a vector quantity.
- Speed is given by the relation:
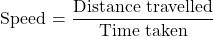 Velocity is given by the relation:
Velocity is given by the relation: 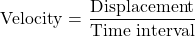
- The speed of an object can never be negative; it can only be zero or positive. The velocity of an object can be negative, positive, or equal to zero.
Q8. Which of the following is true for displacement?
(a) It cannot be zero.
(b) Its magnitude is greater than the distance traveled by the object.
Ans – Neither (a) nor (b) is true
Displacement is the change in position of an object, defined as the shortest distance from the initial position to the final position.
(a) It cannot be zero: This is not true. Displacement can be zero if an object returns to its starting point, making the initial and final positions the same.
(b) Its magnitude is greater than the distance travelled by the object: This is also not true. The magnitude of displacement is always less than or equal to the total distance traveled. It is only equal to the distance traveled if the motion is in a single straight line and direction.
Q9. When will you say a body is in (i) uniform acceleration? (ii) non–uniform acceleration?
Ans –
(i) Uniform acceleration : A body is in uniform acceleration when it travels in a straight line and its velocity changes by equal amounts in equal intervals of time.
(ii) Non–uniform acceleration: A body is in non–uniform acceleration when its velocity changes by unequal amounts in equal intervals of time.
What can you say about the motion of an object whose distance – ‘time graph is a straight line parallel to the time axis?
Ans – The object is at rest.
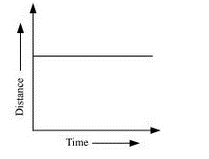
A straight line parallel to the time (x) axis in a distance–time graph indicates that the object’s distance from the origin remains constant as time progresses. This means there is no change in the object’s position over time, which is the definition of being stationary or at rest.
Chapter 8 – Forces and Laws of Motion
Q1. Write the Newton first law of motion.
Ans – A body remains in the state of rest or uniform motion in a straight line unless and until an external force acts on it.
Q2. Write the Newton’s Third Law of Motion.
Ans: Newton’s Third Law of Motion states:
“For every action, there is an equal and opposite reaction.”
Whenever an object exerts a force on another object, the second object exerts a force of equal magnitude but in the opposite direction on the first object. These forces act on different bodies, not on the same body
Example:
- When you push a wall, the wall pushes back with an equal force in the opposite direction.
- When a rocket launches, hot gases are pushed downwards, and the rocket is pushed upwards.
Q3. Why do you fall in the forward direction, when a moving bus brakes to a stop and fall backwards, when it accelerates from rest? Most Important
Ans –
- Every object tries to maintain its state of motion or rest. If an object is at rest, it tends to remain at rest. If an object is in motion, it tends to remain in motion. In a moving bus, a passenger moves along with the bus. As soon as the driver applies the brakes, the bus stops. However, the passenger tries to maintain his state of motion. As a result, a force acts on him in the forward direction.
- Similarly, when a bus starts moving from a state of rest, the passenger falls backward. This is because when the bus starts moving, the inertia of the passenger opposes the forward motion of the bus. Therefore, when the bus moves forward, the passenger falls backward.
Q4. When a carpet is beaten with a stick, dust comes out of it. Explain.
Ans – When a carpet is beaten with a stick, dust comes out of it due to Newton’s first law of motion. The dust particles, initially at rest on the carpet, remain at rest due to their inertia while the carpet moves, causing the dust to separate from the carpet.
Q5. Why is it advised to tie any luggage kept on the roof of a bus with a rope ?
Ans – When a moving bus suddenly stops, the luggage on the roof tends to continue its state of motion and may fall. Also, when the bus suddenly starts from rest, luggage maintains its rest position and may fall backward. So, it is advised to tie any luggage kept on the roof of a bus with a rope.
Q6. A motor car is moving with a velocity of 108 km/hour and it takes 4 second to stop after the brakes are applied. Calculate the force exerted by the brakes on the motor car if its mass along with the passengers is 1000 kg.
Ans – Initial Speed (u) = 108km/h = ![]() = 30m/s
= 30m/s
Final Speed (v) = 0 km/h (because the car stops)
Time (t) = 4 sec
mass (m) = 1000kg
Find acceleration: ![]() =
= ![]() = -7.5 m/s2
= -7.5 m/s2
Find force to using newton second law
F = ma
F = 1000 X (–7.5)
F = – 7500N
Q7. Explain why some of the leaves may get detached from a tree if we vigorously shake its branch. Most Important
Ans – When the tree’s branch is shaken vigorously the branch attain motion but the leaves stay at rest. Due to the inertia of rest, the leaves tend to remain in its position and hence detaches from the tree to fall down.
Q8. Explain, why is it difficult for a fireman to hold a hose, which ejects a large amount of water at a high velocity.
Ans – The water that is ejected out from the hose in the forward direction comes out with a large momentum and equal amount of momentum is developed in the hose in the opposite direction and hence the hose is pushed backward. It becomes difficult for a fireman to hold a hose which experiences this large momentum.
Chapter 9 – Gravitation
Q1. Define Weight. Write the unit of weight.
Ans – Weight is the force with which a body is attracted towards the Earth due to gravity.
Formula: weight = mass x acceleration
Unit of weight:
- In the SI system: Newton (N)
- In the CGS system: Dyne
Q2. Write three differences between the mass of an object and its weight. Most Important
Ans –
- Mass is the amount of matter in an object, whereas weight is the force exerted on that object due to gravity.
- Mass is measured in kilograms (kg); weight is measured in newtons (N).
- Mass does not change with location; weight changes if gravity changes (e.g., on the Moon).
Q3. What are the importance of universal law of Gravitation? Most Important
Ans – The importance of the Universal Law of Gravitation is as follows:
- With the help of this law, we can determine the masses of the Earth, the Sun, etc.
- Using this law, the distances between planets and other celestial bodies can be calculated.
- Due to this force, the Moon revolves around the Earth.
- This law helps us understand the occurrence of tides in the sea due to the Moon and the Sun.
Q4. Why is it difficult to hold a school bag having a strap made of a thin and strong string? Most Important
Ans – It is difficult to hold a school bag with a thin strap, even if the strap is strong, because:
- Pressure is the force applied per unit area. A thin strap has a very small contact area on your shoulder.
- Even though the strap can withstand the weight (force), the pressure on your shoulder becomes very high, causing discomfort or pain.
So, thicker straps are used to spread the force over a larger area, reducing pressure and making it easier to carry the bag.
Q5. An object weight 10 N when measured on the surface of the earth What would be its weight when measured on the surface of the moon?
Ans – The weight of an object depends on the gravitational force acting on it. The gravitational force on the moon is about 1/6th that of the Earth. If the object weighs 10 N on the Earth, its weight on the moon can be calculated as: Weight on the Moon = 1/6th of Weight on Earth
Weight on moon = 10 10 ÷ 6 ≈ 1.67 N
Q6. What do you mean by buoyancy? The volume of 50 g of a substance is 20 cm3. If the density of water is 1 g/cm3, will the substance float or sink?
Ans – Buoyancy is the upward force exerted by a fluid on an object immersed in it, which opposes the weight of the object. It determines whether an object floats or sinks in the fluid.
Mass of substance = 50g
Volume of substance = 20 cm3
Density of water = 1 gcm–3
Calculate density of the substance
Density = 2.5 gcm–3
the substance is denser than water, so it will sink
Q7. A ball thrown up vertically returns to the thrower after 6 seconds. Find : Most Important
(i) The velocity with which it was thrown up.
(ii) The maximum height it reaches
(iii) Its position after 4 seconds.
Ans – Time of flight, T = 6s
Step 1: Initial velocity(u)
Time to go up = time to come down = ![]()
using equation : v=u-gt
At max. height v = 0
0 = u – g.3
u = g.3 = 10 x 3 = 30 m/s
= 30m/s
Step 2: Maximum Height (H)
![]()
H = 45 m
Step 3 : Position after 4 second
Equation : ![]()
![]()
S = 120 – 5.15 = 120 – 80 =40m
S = 40 m above the thrower
Q8. A stone is thrown vertically upward with an initial velocity of 40 m/s. Find the maximum displacement and the total distance covered by the stone ? ( g = 10 m/s2 )
Ans –
Initial Velocity (u) = 40 m/s
Final Velocity at max height (v) = 0
acceleration (a) = -g = -10m/s2
Use this equation:
v2 = u2 + 2as
0 = 402 + 2 (-10)s
20s = 1600
s = 80
Total Distance Covered
The stone goes up 80 m and then comes down 80 m back to the ground.
Total distance = 80 + 80 = 160m
Maximum displacement = 80 m
Q9. How does the force of gravitation between two objects change when the distance between them is reduced to half?
Ans – The gravitational force between two objects is governed by Newton’s Law of Universal Gravitation, which states:
![]()
Let the original distance be r.
If the new distance becomes r/2, then :
![]()
![]()
![]()
Fnew = 4f
Q10. What do you mean by buoyancy?
Ans – The upward force exerted by any fluid (liquid, gas) on an object is known as upthrust or buoyancy.
Q11. Why does an object float or sink when placed on the surface of water?
Ans – The density of the objects and water decides the floating or sinking of the object in water. The density of water is 1 gm/cm3.
- If the density of an object is less than the density of water then the object will float.
- If the density of an object is more than the density of water then the object will sink.
Q12. In what direction does the buoyant force on an object immersed in a liquid act?
Ans – The buoyant force on an object immersed in a liquid acts upwards, i.e. opposite to the direction of the force exerted by the object.
Q13. State the universal law of gravitation.
Ans – The universal law of gravitation states that every object in the universe attracts every other object with a force called the gravitational force. The force acting between two objects is directly proportional to the product of their masses and inversely proportional to the square of the distance between their centers.
Masses of two objects m1 and m2
distance between the two object = r
Force of attracting acting between them =F
universal constant = G
![]()
(6.674×10-11 N.m2/kg2
Q14. What is the acceleration of free fall?
Ans – The acceleration of free fall, denoted by ‘𝑔’, is the constant rate at which objects accelerate downwards due to Earth’s gravity, approximately 9.8 meters per second squared (m/s²)
Q15. A stone is released from the top of a tower of height 19.6 m: Calculate its final velocity just before touching the ground?
Ans – initial velocity, u = 0
Height, h = 19.6 m
Acceleration due to gravity, g = 9.8 m/s2
Using the third equation of motion :
V2 = u2 + 2gh
V2 = 0 + 2 x 9.8 x 19.6
V2 = 384.16
V = 19.6 m/s
Q16. Why is the weight of an object on the moon 1/6 th its weight on earth?
Ans –
- The weight of an object depends on the acceleration due to gravity (g) acting on it.
- The value of acceleration due to gravity on the Moon is one-sixth of that on the Earth.
- Since weight W= mg, the weight of an object on the Moon becomes 1/6th of its weight on the Earth.
Chapter 10 – Work and Energy
Q1. What is Potential energy ? Write its formula. Most Important
Ans – Potential energy is the energy possessed by an object due to its position or configuration.
The electric potential energy formula is ![]() Where UE is the electric potential energy k stands for Coulomb’s constant whereas q1 and q2 stands for charges of the two separate points present in the circuit r stands for distance of the separation.
Where UE is the electric potential energy k stands for Coulomb’s constant whereas q1 and q2 stands for charges of the two separate points present in the circuit r stands for distance of the separation.
Q2. What is Potential energy ? Give two examples of potential energy ?
Ans – Potential energy is the energy possessed by an object due to its position or configuration.
Examples of Potential Energy:
- Water stored in a dam has potential energy due to its height.
- A stretched rubber band possesses potential energy due to its stretched shape.
Q3. What is the kinetic energy of an object? Write an expression for the kinetic energy of an object.
Ans – Kinetic energy is the energy possessed by an object due to its motion. The expression for kinetic energy (KE) of an object with mass m and velocity v is given by the formula: ![]()
Q4. Write the law of conservation of energy.
Ans – The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms.
Q5. The potential energy of a freely falling object decreases progressively. Does this violate the law of conservation of energy ? Why ?
Ans – No, it does not violate the law of conservation of energy. As the object falls, its potential energy decreases while its kinetic energy increases by the same amount, keeping the total mechanical energy constant.
Q6. What is Power ? Write its S.I. unit. Most Important
Ans: Power is defined as the rate at which work is done or energy is transferred.
Power = work/time
S.I. unit is watt
Q7. What is power ? Define 1 watt of power.
Ans – Power is defined as the rate at which work is done or energy is transferred.
One watt is defined as the power produced when one joule of energy is transferred or converted per second.
Q8. Define average power. Most Important
Ans – Average power is defined as the total energy consumed or work done divided by the total time taken
Q9. Look at the activities listed below. Reason out whether or not work is done in the light of your understanding of the term ‘work’:
(a) Suma is swimming in a pond.
(b) Food grains are getting dried in the sun.
(c) An engine is pulling a train.
(d) A sailboat is moving due to wind energy.
Ans – We can analyze each activity using the physics definition of work:
Work is done when a force is applied on an object and the object displaces in the direction of the force
(a) Suma is swimming in a pond
- She applies force on water with her hands and feet, and her body moves forward.
- Work is done because her force causes displacement of her body in the direction of applied force.
(b) Food grains are getting dried in the sun
- The sun provides heat, but no mechanical force is causing displacement of the grains.
- No work is done in the physics sense.
(c) An engine is pulling a train
- The engine exerts a force on the train, causing it to move.
- Work is done because there is force and displacement in the direction of force.
(d) A sailboat is moving due to wind energy
- The wind exerts force on the sails, causing the boat to move.
- Work is done because the force of wind causes displacement in the direction of force
Summary:
- Work is done: (a) Swimming, (c) Engine pulling train, (d) Sailboat moving
- No work is done: (b) Food grains drying in the sun
Q10. What are the various energy transformations that occur when you are riding a bicycle ?
Ans – Muscular Energy to Mechanical Energy:
Mechanical Energy to Kinetic Energy:
- Conservation of Energy:
Kinetic Energy to Heat Energy:
When you are riding a bicycle, several energy transformations take place:
- Chemical energy → Mechanical energy
The chemical energy stored in your muscles (from food) is converted into mechanical energy to pedal the bicycle.
- Mechanical energy → Kinetic energy
The pedaling motion (mechanical energy) moves the bicycle, giving it kinetic energy as it travels forward.
- Mechanical energy → Sound energy
Some energy is transformed into sound, such as the creaking of pedals or wheels.
- Mechanical energy → Thermal energy
Some energy is lost as heat due to friction between tires and the road, chain and gears, and air resistance.
So, riding a bicycle involves chemical → mechanical → kinetic + sound + thermal energy transformations.
Q11. A battery lights a bulb. Describe the energy changes involved in the process.
Ans – When a battery lights a bulb, the following energy transformations take place:
- Chemical energy → Electrical energy
The battery stores chemical energy in its chemicals. When the circuit is completed, this energy is converted into electrical energy that flows through the wires.
- Electrical energy → Light energy
The electrical energy powers the filament of the bulb, which produces light.
- Electrical energy → Thermal energy
Some electrical energy is also converted into heat, which is why the bulb filament becomes hot.
Summary of energy changes:
Chemical energy (in battery) → Electrical energy → Light energy + Heat energy (in bulb)
Q12. Find the energy in KWh consumed in 10 hours by four devices of power 500 W each. Most Important
Ans – Each device has a power of 500 W, which is 0.5 kW.
There are four devices, so the total power is 4 × 0.5 kW = 2 kW.
The energy consumed is calculated using the formula: Energy (kwh) = Power (kW) x Time (h)
Power = 2Kw , Time = 10hours
Energy = 2 x 10 = 20 kWh
Thus, the total energy consumed is 20 kWh.
Q13. An object of mass 15 kg is moving with a uniform velocity of 4 m/s. What is the kinetic energy possessed by the object? Most Important
Ans – Kinetic energy = ½ mv2
Mass = 15kg
Velocity = 4m/s
Kinetic energy = ½ x15 x 42 = 120
Thus, the kinetic energy is 120 Joules.
Q14. An object of mass 40 kg is raised to a height of 5 M above the ground. What is its potential energy? If the object is allowed to fall, find its kinetic energy when it is half–way down. (g = 10 m/sec2)
Ans –
mass (m) = 40kg
Height (h) = 5 m
Acceleration due to gravity (g) = 10 m/s2
Step1: Potential Energy at the top
PE = mgh
PE = 40 x 10 x 5
PE = 2000J
Step 2: Kinetic Energy at half–way down
When the object falls halfway, height above the ground h’ = 2.5m
Potential energy at that height:
PEhalf = mgh‘ = 40 x 10 x 2.5 = 1000J
Total mechanical energy is conserved,
Total Energy = PE + KE = 2000J
KEhalf = Total energy – PEhalf
KEhalf = 2000 – 1000 = 1000J
Potential energy at top = 2000 J
Kinetic energy halfway down = 1000 J
Q15. Two girls each of weight 200 N, climb up a rope through a height of 4 m. A takes 10 seconds while B takes 30 seconds to accomplish this task. What is the power expanded by each girl ?
Ans –
![]()
Step 1: Calculate Work Done
Work done in climbing = weight × height
Weight = 200 N
Height = 4 m
Work = 200×4 = 800 J
Step 2: Calculate Power for Each Girl
Girl A:
Time = 10 s
![]()
Girl B:
Time = 30 s
![]()
- Power expended by Girl A = 80 W
- Power expended by Girl B = 26.67 W
Q16. Find the energy possessed by an object of mass 10 kg, when it is at a height of 6 m, above the ground. ( g = 9.8 m/sec2 )
Ans – Find the energy possessed by an object of mass 10 kg, when it is at a height of 6 m, above the ground. ( g = 9.8 m/sec2 )
Q17. A bulb consumes 500 J of electrical energy in 10 sec. What is its power?
Ans – To find the power of the bulb, use the formula:
![]()
Given that the bulb consumes 500 J of electrical energy in 10 seconds, the power is calculated as follows:
P = 500 J / 10 s = 50 W.
Thus, the power of the bulb is 50 watts.
Q18. A porter lifts a luggage of 15 kg from the ground and puts it on his head 1.5m above the ground. Calculate the work done by him on the luggage.
Ans – To calculate the work done by the porter, we use the formula:
Work = Force × Distance (in the direction of force)
Force = Weight= m⋅g
Where:
mass of luggage (m) = 15kg
height lifted (h) = 1.5 m
Acceleration due to gravity (g) = 9.8m/s2
Step–by–step Calculation:
Weight of luggage:
= m⋅ g = 15×9.8 = 147N
Work done:
W = F⋅h = 147 × 1.5
W = 220.5 J
Work done by the porter = 220.5 joules
Q19. Calculate the work required to be done to stop a car 1500 kg moving at a velocity of 60 km/h.
Ans – We are asked to calculate the work required to stop a car. The work done to stop a moving object is equal to the change in its kinetic energy.
Step 1: Write down the given data
![]()
The work required to stop the car is:
W = ΔKE = KEfinal – KEinitial
KEfinal = 0
![]()
The negative sign indicates that the work is done against the motion, but we’ll consider the magnitude.
Step 2: Convert velocity to meters per second
![]()
Step 3: Calculate kinetic energy
![]()
![]()
The work required to stop the car is 208,500 joules.
Q20. A freely falling object eventually stops on reaching the ground. What happens to its kinetic energy?
Ans – When an object falls freely towards the ground, its potential energy decreases and kinetic energy increases. As the object touches the ground, all its potential energy gets converted into kinetic energy. As the object hits the hard ground. All its kinetic energy gets converted into heat energy and sound energy. it can also deform the ground depending upon the nature of the ground and the amount of kinetic energy possessed by the object.
Chapter 11 – Sound
Q1. How does the sound produced by a vibrating object in a medium reach your ear?
Ans – Air is the commonest material through which sound propagates. When vibrating objects, like prongs of a tuning fork move forward, they push the molecules of the air in front of them. This in turn compresses the air, thus creating a region of high pressure and high density called compression. This compression in the air travels forward. When the prongs of the tuning fork move backward, they create a region of low pressure in the air, commonly called rarefaction.
This region has low pressure, low density, and more volume. As the tuning fork continues to vibrate, the regions of compression in the air alternate with the regions of rarefaction. These regions alternate at the same place. The energy of vibrating tuning fork travels outward. This energy which reaches the ears, makes the eardrums to vibrate and thus we hear sound.
Q2. Why are sound waves called mechanical waves ?
Ans – Some mechanical energy is required to make an object vibrate. Sound energy cannot be produced on its own. The mechanical energy Of vibrating object travels through a medium and finally reaches the ear. Therefore, the sound waves are called mechanical waves.
Q3. Define the following terms: Most Important
(i) Time period
(ii) wavelength
(iii) Amplitude
OR
What are time period and amplitude of a sound wave ? Most Important
Ans – (i) Time Period
The time period of a sound wave is the time taken to complete one full vibration or cycle.
It is measured in seconds (s).
It is inversely related to frequency:
![]()
(ii) The distance between two consecutive compressions (C) or two consecutive rarefactions (R) is called the wavelength.
(iii) Amplitude
- The amplitude of a sound wave is the maximum displacement of particles from their rest position during vibration.
- It determines the loudness of the sound — greater amplitude means louder sound.
- It is measured in meters (m) in wave motion.
Q4. Give two practical applications of reflection of sound waves.
Ans –
Stethoscope: The sound of a heartbeat reaches the doctor’s ears through multiple reflections of sound, allowing for accurate medical diagnosis.
SONAR: Sound Navigation and Ranging uses sound waves to measure the distance and speed of underwater objects, aiding in navigation and exploration
Q5. Distinguish between loudness and intensity of sound. Most Important
Ans –
Loudness: Subjective perception of sound intensity by the human ear, influenced by factors like frequency and duration. Measured in decibels (dB).
Sound Intensity: Objective measurement of sound energy carried by sound waves per unit area, measured in watts per meter square (W/m²).
Q6. The time taken by the wave for one complete oscillation of the density of the medium is called the _______.
Ans – time period.
Q7. The sound which is produced due to a mixture of several frequencies is called and is pleasant to listen.
Ans – The sound which is produced due to a mixture of several frequencies is called a note and is pleasant to listen to.
Q8. Which wave property determines (a) loudness, (b) Pitch ?
Ans – (a) The amplitude of the wave determines the loudness; more the amplitude of a wave, more is the loudness produced.
(b) The pitch is determined by the frequency of the wave. Higher the frequency of a wave more is its pitch and shriller is the sound.
Q9. How are the wavelength and frequency of a sound wave related to its speed ?
Ans – Speed of sound Frequency x Wavelength
Q10. What is the range of frequencies associated with (a) Infra sound ? (b) Ultrasound ?
Ans – (a) Infra sound : Sound waves between the Frequencies 1 and 20 Hz.
(b) Ultrasound : Sound waves of the frequencies above 20,000 Hz.
Q11. What is sound and how is it produced ?
Ans – Sound is mechanical energy which produces a sensation of hearing. When an Object is set into vibrations, sound is produced.
Q12. Give two practical applications of reflection of sound waves.
Ans –
- Reflection of sound is used in megaphones, horns and musical instruments such as trumpets and shehnai.
- It is used in stethoscope for hearing patient’s heartbeat.
- Ceilings of the concert halls are curved, so that sound after reflection reaches all comers of the hall.
Q13. Explain how the human ear works.
Ans – The outer ear is called “pinna’. It collects the sound from the surroundings. The collected sound passes through the auditory canal. At the end of the auditory canal there is a thin membrane called the eardrum or the tympanic membrane. When a compression of the medium reaches the eardrum the pressure on the outside of the membrane increases and forces the eardrum inward. Similarly, the eardrum moves outward when a rarefaction reaches it. In this way the eardrum vibrates. The vibrations are amplified several times by three bones (the hammer, anvil and stirrup) in the middle ear. The middle ear transmits the amplified pressure variations received from the sound wave to the inner ear. In the inner ear, the pressure variations are turned into electrical signals by the cochlea. These electrical signals are sent to the brain via the auditory nerve and the brain interprets them as sound.
Chapter 12 – Improvement in Food Resources
Q1. How animal husbandry practices are beneficial for farmers? Most Important
Ans –
- Improvement of Breeds: Good practices lead to the improvement of breeds of domesticated animals, enhancing their productivity and quality.
- Increased Yields: These practices contribute to increases in yields of foodstuffs such as milk, meat, and eggs, which are essential for farmers’ income.
- Proper Management: Farmers benefit from proper management of animals, which includes providing clean shelter, feed, and protection against diseases.
- Sustainable Resource Management: Good animal husbandry practices promote sustainable resource management, minimizing environmental impact and conserving natural resources.
- Diversification of Income Streams: By diversifying their farming practices, farmerscan create multiple income sources, reducing the risk of financial loss.
These benefits collectively contribute to the overall success and sustainability of farmers’ businesses.
Q2. How are fish obtained ? Most Important
Ans – Fish can be obtained through capture fishing and culture fishery.
Q3. For increasing production, what is common in poultry, fisheries and bee– keeping ?
Ans – For increasing production, a common practice in poultry, fisheries, and beekeeping is providing proper care, nutrition, and a controlled environment.
Explanation:
- Poultry: Balanced feed, clean housing, and disease prevention increase egg and meat production.
- Fisheries: Proper feeding, pond management, and water quality control improve fish growth and yield.
- Beekeeping: Providing healthy hives, good flowers/nectar sources, and disease control increases honey production.
Summary: Careful management, feeding, and hygiene are common methods to enhance production in all three sectors.
Q4. Write about composite fish culture.
Ans – Composite fish culture is a sophisticated aquaculture technique that involves the simultaneous cultivation of compatible fish species within a single aquatic environment. This method maximizes fish yield by utilizing various feeding zones and ensuring different species occupy distinct ecological niches, minimizing competition for food and space.
Key points include:
- Technological Advantages
- Maximized Yield
- Market Demand
- Environmental Sustainability
Q5. What are infectious diseases? Give one example.
Ans – Infectious diseases are diseases that are caused by pathogenic microorganisms such as bacteria, viruses, fungi, or parasites and can spread from one person to another, or from animals to humans.
Key points:
- They are contagious or transmissible.
- Spread can occur through air, water, food, direct contact, or vectors like mosquitoes.
- Examples include influenza, tuberculosis, malaria, and chickenpox.
In short, infectious diseases are illnesses caused by microorganisms that invade the body and multiply, leading to symptoms.
Q6. What is pasturage and how is it related to honey production ?
Ans – Pasturage refers to the areas where bees can forage for nectar and pollen, which are essential for honey production. It is crucial because the quality and variety of pasturage directly influence the health of bee colonies and the taste, quality, and quantity of honey produced.
Q7. What is soil erosion ?
Ans – Soil erosion is the process of removing the top layer of soil from the land, primarily caused by natural forces like water and wind, as well as human activities such as deforestation and agriculture.
Q8. Write two common management practices of animal husbandry and poultry farming.
Ans – Here are two common management practices in animal husbandry and poultry farming:
- Proper feeding and nutrition – Providing balanced and adequate feed to ensure growth, health, and productivity of animals and poultry.
- Cleanliness and hygiene – Maintaining clean shelters, proper ventilation, and regular removal of waste to prevent diseases and infections.
Q9. How do biotic and abiotic factors affect crop production?
Ans – Factors responsible for loss of grains, during storage and production are:
(a) Biotic factors like rodents, pests, insects, etc.
(b) Abiotic factors like temperature, humidity, moisture, etc.
Combination of both biotic and abiotic factors causes :
- infestation of insects
- weight loss
- poor germination ability
- degradation in quality
- discolouration
- poor market price
Q10. What are macro–nutrients and why are they called macro–nutrients? Most Important
Ans – Macro–nutrients are the essential elements which are utilised by plants in large quantities. Many macro–nutrients are required by the plants for the following functions:
- As the constituent of protoplasm
- N, P, S are present in proteins
- Ca is present in cell wall
- Mg is important constituent of chlorophyll
Q11. How do plants get nutrients?
Ans – Plants get nutrients from air, water and soil. There are, sixteen nutrients essential for the growth of plants. Carbon and Oxygen are supplied by water. The remaining thirteen nutrients are supplied by soil.
Q12. What factors may be responsible for losses of grains during storage?
Ans – The factors responsible for losses of grains during storage are:
- Abiotic factors like moisture (present in food grains), humidity (of air) and temperature.
- Biotic factors like insects, rodents, birds, mites, bacteria and fungi.
Q13. What management practices are common in dairy and poultry farming?
Ans –
- Shelter: Dairy animals and poultry birds require proper shelter, i.e., well designed dairy and hygienic shelter.
- Feeding: To get good yield of food product, proper feed is provided to dairy animals and poultry birds.
- Caring for animal health: Animal and birds must be protected from diseases caused by virus, bacteria or fungi.
Q14. What are the advantages of inter–cropping and crop rotation?
Ans – Advantages of using inter–cropping:
- It helps to maintain soil fertility.
- It increases productivity per unit area.
- Save labour and time.
- Both crops can be easily harvested and processed separately.
Advantages of using crop rotation:
- It improves the soil fertility.
- It avoids depletion of a particular nutrient from soil.
- It minimise pest infestation and diseases.
- It helps in weed control.
- It prevents change in the chemical nature of the soil.
Q15. Name two greenhouse gases.
Ans – Carbon Dioxide, CFC
Q16. Why do we need food?
Ans – We need food because it:
- Provides energy to do work and carry out daily activities.
- Helps in growth and development of the body.
- Repairs damaged tissues and maintains body functions.
- Protects the body from diseases and keeps us healthy.